Part 2: measuring lithium and other uses for it
In part one of this blog series, we discussed the use of lithium in understanding geochemical processes. In this part, we look at the use of lithium in a completely different application area – the nuclear power sector. We also take a look at how lithium is analyzed, with some focus on isotope ratio measurement.
Actually, there are several applications for lithium in the nuclear power sector. The most well-known application is its use in hydroxide form in Pressurized Water Reactors (PWRs). It’s added to the primary coolant at about two parts per million, where it helps to minimize the corrosive effects of the boric acid (a much more major constituent of the coolant). But does the isotope matter… absolutely it does!

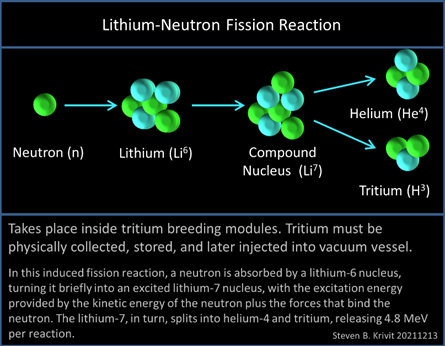
Unfortunately, if you bombard 6Li with neutrons (which is what happens in a nuclear reactor), it has a very high neutron cross section and through fission processes releases tritium. The fact that this is how tritium is enriched for nuclear bombs tells you how much of a Very Bad Thing this is. Therefore, you need 7Li for your nuclear reactor, and naturally this poses problems both for isotope separation and for isotope measurement.
World demand for Li-7 in these PWR cooling systems is about 1,000 Kg per year, including about 400 kg annually for the 65 PWRs being operated in the USA. And where does the 7Li come from? These days mostly from Russia, and it is suspected that it is produced as a as a by-product of enriching 6Li to produce tritium for thermonuclear weapons. It’s all pretty circular really, isn’t it?!
As well as lithium hydroxide for PWRs, lithium is also going to be required in the future in fluoride salt form. This is for a very different kind of nuclear reactor – known as Molten Salt Reactors (MSRs). This reactor type is not in common commercial use yet, but it is coming soon. Here the lithium salt is a primary part of the reaction (typically it has the primary fuel dissolved in it), and it is required in much larger quantities and at extremely high purity levels (it needs to be very pure 7Li, as in up to 99.995%). Here is a great link to an online article about the use of lithium in this industry: link here
We’ve established that lithium is an important metal in several application areas, and that the isotopes matter as well. But how are they measured? You can get a reasonably precise measurement of the isotope ratio using a single collector quadrupole ICP-MS or ICP-TOF. In many applications this would be sufficient. But for some geochemical processes, and absolutely for Molten Salt Reactor applications, an isotope ratio instrument would be required to meet the desired precision.
If precision is your goal, either MC-ICP-MS or TIMS would be your technique of choice. In the early days of isotope ratio measurement, TIMS was used for lithium isotope ratios. Other than the complicated sample prep and chemistry, it worked reliably well and there is a wealth of published data available out there. However, the advent of MC-ICP-MS really made a big difference. As with many isotope systems, typically the sample could be measured with significantly less sample preparation and significantly faster. MC-ICP-MS quite quickly became the dominant technique for lithium isotope ratio measurement. So, is there still a case for TIMS?
I used MC-ICP-MS to analyze lithium a number of times in my early days at VG Elemental, using both the Plasma 54, and its replacement – the VG Axiom. Turns out it is not quite as straightforward as I thought. Firstly, because of the low molecular weight of lithium, and the relatively large difference in mass between 6Li and 7Li, I found the mass bias to be a lot less stable than the heavier elements I was used to measuring. Correcting for a drift in mass bias also wasn’t easy, unless you resorted to sample-standard bracketing (which slows down the analysis somewhat).

Furthermore, the instruments I used tended to be less efficient at the lower part of the mass range, meaning the sensitivity for lithium was only a fraction of what it was for uranium. (Side note – this was not the case for all MC-ICP-MS and could have been a function of interface design).
And to top it off, I also found that the fairly primitive sample introduction system I was using at the time had a quite pronounced memory for lithium, meaning if you didn’t wait long enough for the previous sample to wash out then you got the wrong number. These factors conspired to seriously limit the accuracy and precision I was able to achieve. From memory (and this is the 90’s we’re talking about, so go easy on me) 0.1% RSD external precision after ten measurements was pretty normal – very weak data when compared to heavier isotope systems such as hafnium where 10 to 50 times better precision was commonplace.
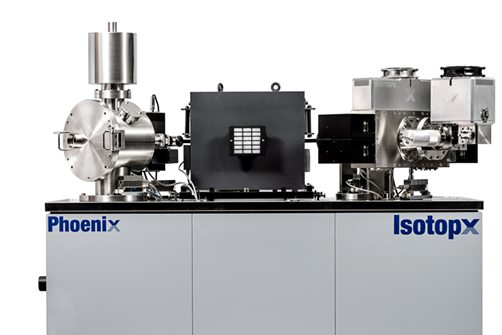
This is where TIMS has a part to play. For ultimate precision, TIMS is still considered the “gold standard”, obviously with the proviso that you can stomach the sample prep time and analysis time! I note several recent publications that discuss lithium isotope ratio measurements at a much better precision than what I’ve quoted from my Axiom days. And with emerging applications in both the geochemical sector and nuclear sector demanding better precision, perhaps its future is secure after all.
If you’ve got any thoughts, questions or comments about lithium and the measurement of its isotopes, please let me know, I’d be keen to hear (Stephen.guilfoyle@isotopx.com).
