I recently attended the 14th International Symposium on Nuclear and Environmental Radiochemical Analysis (aka ERA14, link here). I found this a fascinating insight into the breadth of applications where radiochemical measurements can help science. I’d naturally assumed the meeting would include major focus on nuclear forensics, but the additional focus on medical applications, fuel reprocessing and the underlying radiochemistry was very interesting.
However, the biggest surprise was how much focus was on decommissioning. Like many scientists I’ve been aware for some time that the decommissioning of nuclear power stations was a challenging, costly and time-consuming task. But the sheer scale of the efforts required, and the breadth of skills required to tackle this necessary task really amazed me.
As ever, please note that this blog is an entirely personal one and in no way reflects the views of Isotopx. Anything that involves the nuclear sector is controversial and easily politicized and that is not my intention here. Feel free to contact me if you disagree, can correct me on any of the statements I make, or simply have additional comments (Stephen.guilfoyle@isotopx.com)
The ERA14 meeting was fully international, but due to the location (York, UK) there were quite a number of presentations given by scientists working with UK facilities, including Dounreay, Sellafield and Dungeness. At the meeting I therefore learned a lot about the UK governance of these processes, most especially the Nuclear Decommissioning Authority (link). This body is responsible for the overarching management of decommissioning. Their mission statement is “We’re charged, on behalf of government, with the mission to clean-up the UK’s earliest nuclear sites safely, securely and cost effectively”. Quite a challenge!

Dungeness A reactor gives us an excellent opportunity to understand the magnitude of the decommissioning operation. This reactor began producing power in 1966 and after 40 years of power generation was switched off in 2006. The “defueling” took place in the following few years. It’s hard to imagine how difficult this particular job must have been – removing reactor fuel from a core must surely be one of engineering’s most challenging tasks. But that’s not the end of it of course.
A multitude of buildings, vehicles, items… just huge amounts of “stuff”… all needs checking and channeling into the correct pathway for either safe disposal or recycling. And to think all of this needs doing in an environment that necessitates some of the most stringent safety precautions in the country. In fact much of this activity doesn’t even begin for decades after the reactor is defueled – simply to allow radiation levels to drop (at least as far as the shorter-lived isotopes are concerned). This process is known as the “care and maintenance stage”, and in the case of Dungeness won’t be completed until 2090 or thereabouts. These days it’s hard to think of any other engineering project that is begun with an end date multiple generations in the future (source: Wikipedia).

For some of the really old nuclear sites the task can be even larger. Sellafield is probably the most well-known UK nuclear site, and it has an extremely complex history involving enrichment of nuclear material for weapons, through nuclear power generation, eventually leading to the reprocessing of spent nuclear fuel material from home and abroad. It’s a large site, with over 1,000 buildings, so ensuring full traceability of every item that could conceivably have been contaminated is a Herculean task. As the oldest of the UK’s legacy nuclear sites, it is arguable the least well documented. It’s also had a colorful history, with the well-known fire of 1957 not helping its cause at the time (nor helping the cause for nuclear power in the UK in general).
At the ERA14 meeting I spoke to several people involved with the decommissioning at Sellafield and heard fascinating anecdotal accounts of badly labelled (or unlabelled) containers or drums that have been waiting for examination for many years. The notorious storage ponds at Sellafield are one of the highest priority clean-up jobs, but bearing in mind much of the waste in there is of unknown origin, the clean-up is slow and difficult. I asked why it wasn’t all done remotely by clean-up robots and was told that any movement in the tanks can dislodge the sludge at the bottom of the pond, reducing visibility to zero until the sediment settles back down to the bottom, hours or even days later.

But how does this all relate to isotope ratio mass spectrometry? It’s back to that bit about ensuring we know what’s contaminated, how much it is contaminated, and what it is contaminated with. Scientists and engineers use a suite of tools for this, including instrumentation that relies on radiometric (counting) techniques, and also mass spectrometry-based solutions determining the elements and/or isotopes present.
When would isotope ratio MS be used? Often times it is when there is only a very small sample and the origin needs to be determined. For example, if a single particle of radioactive material is found in the proximity of a nuclear site (probably found via radiometry) then mass spectrometry can be used to determine exactly what radionuclides are present in the single particle. An excellent and recent review of the use of mass spectrometry for decommissioning can be found here.
What are the benefits of using mass spectrometry as compared to radiometric techniques? Speed of analysis is one obvious benefit, especially in the case of very long half life radionuclides, this can be a major challenge for radiometric techniques.
And what about those isotope ratios? Using isotope ratio measurement techniques with simultaneous detection (e.g. TIMS and MC-ICP-MS) would allow the scientists to determine with some precision what the isotope ratios are for even very small or low abundance samples. This would give the analyst information about the origin of the sample (where in the fuel cycle) or even the age of the sample.
For example, 59Ni and 60Co could originate in the steel casing of the core, this becomes activated during reactor operation. And 41Ca is likely to come from concrete shielding. It gets more complicated with the actinides, they could be fission products from fuel or of course by-products from the fuel following neutron capture. Typically (you’d hope!) these radionuclides are present outside the reactor core in only very small amounts, adding to the analytical challenge of measuring them.
The last generation or so has seen big steps in the analytical capability of isotope ratio mass spectrometers to the point that even picogram-level measurements can be made of certain trace actinides. This has huge implications for a number of applications. This includes nuclear decommissioning and the perhaps more topical catch-all “nuclear forensics”, a phrase now used commonly in the media to refer to identifying the source of nuclear contamination from reactors, weapons test, and accidents such as Chernobyl and Fukushima.
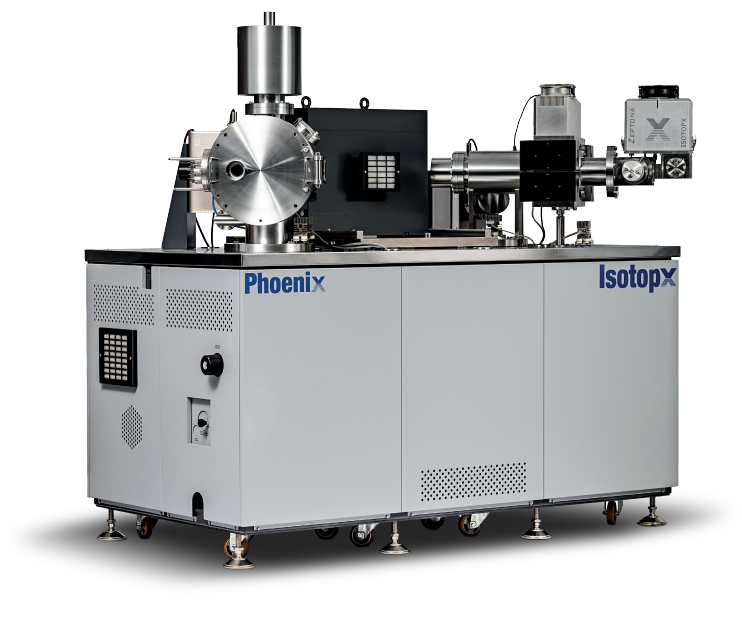
Our latest version of Phoenix TIMS, for example, can be fitted with up to 12 ion counting channels. It’s ideal for these applications where multiple isotopes at trace levels need to be determined using as little analysis time as possible to maximize available sample. We’ve also managed to reduce one of the largest sources of error in these measurements, namely the intercalibration between Faraday detectors (inherently very stable) and the ion counting detectors that have more variability over time and several potential sources of inaccuracy. We do this using our ATONA Faradays that have a much wider linear dynamic range including a large overlap with the ion counting detectors. You can read more about this by clicking here.
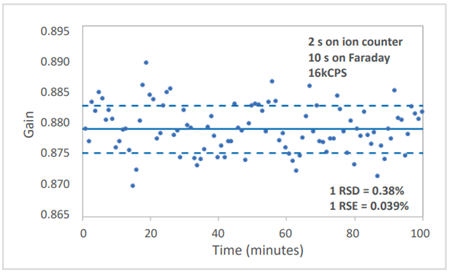
An example of the stability of the Faraday to ion-counter measurement can be seen in the image below.
Isotope ratio mass spectrometry is widely used and well known in the Earth sciences, particularly for geochronology. But it’s just as valuable in the nuclear sciences and will remain a useful and powerful technique for the applications discussed in this article. If you have a comment about this post, or wish to point out an error or addendum, please let me know (Stephen.guilfoyle@isotopx.com).
